Safeguarding the Lifeblood of Pharma: Why Data Protection Matters
Data protection services for pharma are specialized security measures designed to safeguard sensitive information in pharmaceutical companies. These services help protect patient data, research findings, intellectual property, and ensure regulatory compliance.
For pharmaceutical companies seeking immediate guidance, here are the essential data protection services:
| Service Type | Purpose | Key Benefits |
|---|---|---|
| Data Protection Officer (DPO) Services | Ensure regulatory compliance and oversee data governance | Reduces compliance risks and penalties |
| Data Mapping & Classification | Identify and categorize sensitive data across systems | Improves visibility and control of data assets |
| Data Protection Impact Assessments (DPIAs) | Evaluate and mitigate risks in high-risk processing | Prevents breaches and ensures GDPR compliance |
| Cross-Border Transfer Solutions | Secure data sharing across international boundaries | Maintains compliance with regional regulations |
| Anonymization & Pseudonymization | Protect patient data while preserving research value | Balances data utility with privacy requirements |
The pharmaceutical industry faces a double dose of cyber threats \u2013 from both profit-driven criminals and nation-state actors. With data breach costs averaging over $5 million in the pharmaceutical sector (the third most expensive of any industry), the stakes couldn’t be higher.
Over 83% of pharma companies now use cloud environments, creating new vulnerabilities as sensitive data moves beyond traditional security perimeters. Meanwhile, intellectual property leaks cost the industry approximately 4% of its annual revenue.
The challenge is clear: how can pharmaceutical companies protect their most valuable assets \u2013 patient data and intellectual property \u2013 while meeting strict regulatory requirements like HIPAA, GDPR, and FDA 21 CFR Part 11?
At Titan Technologies, we understand that effective data protection isn’t just about compliance \u2013 it’s about safeguarding the very pillars that support pharmaceutical innovation and patient trust.
Data protection services for pharma glossary:
- data protection for financial services
- data protection governance risk management and compliance
- data protection officer services
Understanding the Data Protection Challenges and Regulations in Pharma
Picture yourself walking through a modern pharmaceutical company. In every department, sensitive data flows constantly—from research labs to clinical trials, from patient records to manufacturing processes. This complex ecosystem creates unique challenges for protecting information that’s not just valuable, but often life-changing.
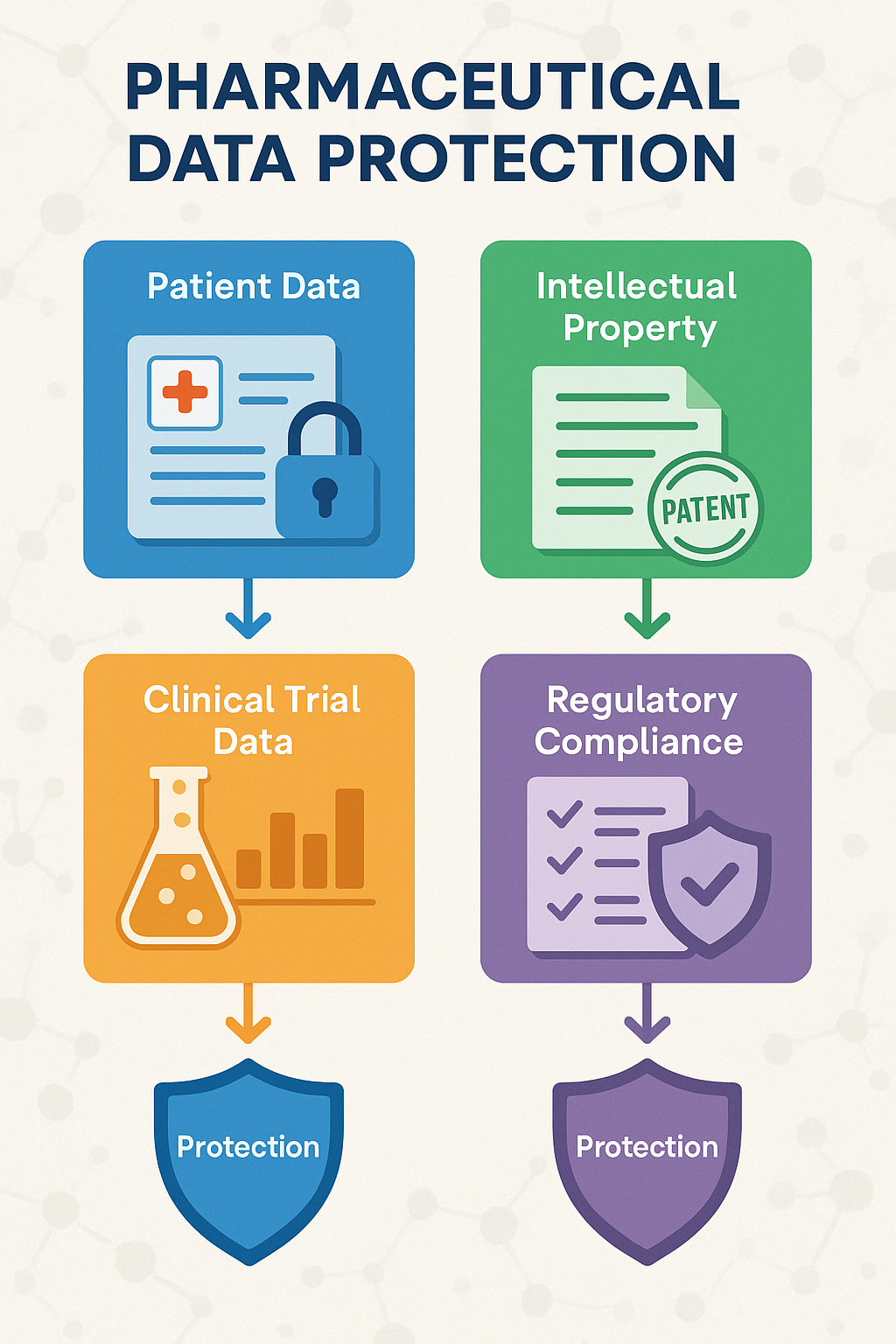
The Data Protection Trifecta: Patient Data, IP, and Regulatory Compliance
When we talk with our pharmaceutical clients in New Jersey, we often describe what we call the “data protection trifecta”—three critical categories of information that need robust protection:
First, there’s patient health information. Think about it: clinical trial data, detailed medical histories, genetic information, and treatment records all contain incredibly personal details about real people. This information isn’t just sensitive—it’s sacred.
Second, we have intellectual property. Our pharmaceutical clients invest billions in developing new drugs and treatments. Their formulations, research methodologies, and findies represent years of work and massive financial investments. As one pharmaceutical executive from a major New Jersey company told us: “If data has business value, it is inherently at risk. In our industry, virtually all data has significant value—to us, to our competitors, and unfortunately, to cybercriminals as well.”
Finally, there’s operational data—the behind-the-scenes information about manufacturing processes, quality control, and supply chains. This might seem less sensitive, but in the wrong hands, it could disrupt production or compromise product safety.
The Regulatory Maze: Navigating Compliance Requirements
If protecting all this data wasn’t challenging enough, pharmaceutical companies must also steer a complex web of regulations. It’s like trying to solve a puzzle where the pieces keep changing shape:
HIPAA sets the standards for protecting patient health information in the United States. The penalties for violations aren’t small—they range from $100 to $50,000 per violation, with a maximum of $1.5 million per year. That’s serious business. Learn more about HIPAA
Then there’s GDPR, the European Union’s comprehensive data protection law. If you handle EU citizens’ data (and most global pharma companies do), you need to comply—or face penalties up to €20 million or 4% of global annual revenue, whichever is higher.
FDA 21 CFR Part 11 focuses specifically on electronic records and signatures in the pharmaceutical industry. It ensures digital records are as trustworthy and reliable as paper ones.
Add to this the CCPA and other state privacy laws creating a patchwork of regulations across the U.S., plus industry-specific guidelines like Good Clinical Practice (GCP), and you’ve got a compliance challenge that would make anyone’s head spin.
The global nature of pharmaceutical operations complicates things further. A recent case highlighted this when a French laboratory software provider was fined 1.5 million euros under GDPR for a data breach affecting nearly 500,000 individuals from 28 laboratories. One breach, multiple countries, massive consequences.
The Shifting Landscape: From On-Premises to Cloud
Remember when all your data lived safely within your company’s walls? Those days are gone. Today, over 83% of pharmaceutical companies use cloud environments, with mentions of cloud computing increasing by 65% in annual reports.
This shift to the cloud creates new vulnerabilities that our data protection services for pharma are designed to address:
Data Drift happens when sensitive R&D or clinical trial data “drifts” to unauthorized locations. We’ve seen cases where researchers accidentally stored confidential trial data in personal cloud accounts, creating serious security risks.
Misconfigurations can be devastating. One major pharmaceutical company faced a massive breach when a misconfigured cloud storage bucket exposed critical drug safety data—a simple technical error with enormous consequences.
Shadow IT develops when departments adopt unauthorized applications or services, creating security blind spots. We’ve helped clients find and secure dozens of unauthorized cloud services that were processing sensitive data without proper controls.
Cross-Border Data Flows occur when cloud services store or process data across international boundaries. This creates compliance headaches, especially with regulations like GDPR that restrict data transfers.
At Titan Technologies, we’ve guided pharmaceutical clients throughout Central New Jersey—from Princeton’s research corridors to New Brunswick’s healthcare hub—through these challenges. Our data protection services for pharma don’t just check compliance boxes; they build comprehensive security that protects what matters most: the data that drives innovation and improves lives.
Implementing Effective Data Protection Services for Pharma
Let’s face it – protecting data in the pharmaceutical world is like trying to guard the crown jewels while they’re on display. It’s valuable, everyone wants to see it, but not everyone should touch it. Implementing robust data protection services for pharma isn’t just about checking compliance boxes; it’s about creating a security ecosystem that protects your company’s most valuable assets.
Step 1: Comprehensive Data Mapping and Classification
Before you can protect something, you need to know where it is. Think of data mapping as creating a treasure map of all your sensitive information.
When we worked with a mid-sized pharmaceutical company in Princeton last year, their IT director was shocked when our mapping exercise revealed sensitive patient data scattered across 17 different systems—several of which weren’t even on their official inventory!
“It was like finding rooms in our house we didn’t know existed,” the director told us, “and some of them had the doors wide open.”
Good data mapping includes creating a thorough inventory of all data assets, categorizing information based on sensitivity levels, and documenting how data flows throughout your organization. This visibility is the foundation of everything that follows.
Step 2: Conducting Data Protection Impact Assessments (DPIAs)
DPIAs aren’t just paperwork—they’re your early warning system. When you’re processing sensitive patient data or implementing new technologies, a DPIA helps you spot potential privacy issues before they become problems.
One of our pharmaceutical clients in Edison avoided a potential GDPR nightmare when their DPIA revealed that a new clinical trial platform would have transferred patient data to servers outside approved regions. Catching this early saved them from potential fines that could have reached millions.
A proper DPIA examines the purpose of your data processing, evaluates risks to individuals’ privacy, implements mitigation measures, and documents your decision-making process. It’s like having a safety inspection before opening a new facility—a little preparation prevents major headaches later.
Learn more about our Data Protection Management services
Step 3: Implementing Technical Safeguards
The technical side of data protection services for pharma is where the rubber meets the road. Modern pharmaceutical data protection requires multiple layers of security:
Strong encryption is non-negotiable for pharmaceutical companies. When a client in Trenton implemented our recommended encryption protocols, they not only secured their valuable research data but also streamlined their compliance reporting. Their compliance officer told us, “For the first time, I’m not losing sleep before audits.”
Access controls should follow the principle of least privilege—give people access only to what they absolutely need. We’ve seen too many cases where broad access permissions led to data leaks. One pharmaceutical researcher shouldn’t have access to all clinical trials, just as your dermatologist doesn’t need to know about your knee surgery.
Cloud security deserves special attention since so much pharmaceutical data now lives in the cloud. Regular audits of cloud configurations, implementing security monitoring tools, and establishing clear data loss prevention policies are essential steps that can prevent catastrophic breaches.
Data Protection Services for Pharma: Key Strategies
Beyond the foundation, there are several strategies that can take your pharmaceutical data protection from adequate to excellent.
Regular security audits catch vulnerabilities before hackers do. We recommend quarterly vulnerability assessments for our pharmaceutical clients, with more comprehensive penetration testing annually. These tests often reveal surprising security gaps—like when we finded a client’s laboratory systems were still using default passwords from installation. Oops!
Developing incident response plans is like having a fire drill for your data. You hope you’ll never need it, but if trouble strikes, you’ll be glad you prepared. A well-crafted plan clearly defines who does what during a security incident, how to contain and eliminate threats, and how to communicate with stakeholders.
When a ransomware attack hit one of our pharmaceutical clients in Red Bank, their team knew exactly what to do because we had run tabletop exercises just months earlier. They isolated affected systems within 30 minutes, preventing the encryption from spreading to their critical research databases. As their CIO told us afterward, “That practice run saved our company.”
Employee training might sound boring, but it’s your secret weapon. The most common entry point for attackers isn’t some sophisticated hack—it’s a tired employee clicking a phishing link or using a weak password. Regular, engaging security awareness training turns your team from a vulnerability into your first line of defense.
The Role of Data Protection Officers in Data Protection Services for Pharma
Think of a Data Protection Officer (DPO) as your data privacy quarterback—coordinating your team’s efforts and making critical calls when needed. For many pharmaceutical companies, having a DPO isn’t just good practice—it’s required by regulations like GDPR.
A pharmaceutical DPO wears many hats: compliance monitor, risk manager, trainer, regulatory liaison, and advocate for individual privacy rights. When one of our clients in New Brunswick appointed a dedicated DPO, they saw compliance violations drop by 68% in the first year. Not coincidentally, their data breach insurance premiums also decreased.
Should you hire an in-house DPO or outsource the role? Both approaches have merit. An in-house DPO develops deep knowledge of your specific operations, while an outsourced DPO brings specialized expertise and independence. For mid-sized pharmaceutical companies, the outsourced model often provides the best balance of expertise and cost-effectiveness.
Here at Titan Technologies, we’ve provided outsourced DPO services for pharmaceutical companies throughout Central New Jersey for years. Our DPOs become extensions of your team, bringing specialized knowledge without the overhead of a full-time executive position.
For more information on regulatory requirements for DPOs, the Information Commissioner’s Office (ICO) offers excellent guidance that we frequently recommend to our clients.
Effective data protection services for pharma aren’t just about avoiding fines or preventing breaches—though they certainly do that. They’re about maintaining the trust that patients, partners, and regulators place in your organization. In an industry built on improving human health, protecting the data that makes that mission possible is both a practical necessity and an ethical obligation.
Leveraging Technology and Best Practices for Improved Data Security
The digital change of the pharmaceutical industry has created both opportunities and challenges when it comes to protecting sensitive data. Today’s data protection services for pharma go far beyond traditional security measures, embracing cutting-edge technologies that offer stronger safeguards for valuable pharmaceutical information.
Cloud Security for Pharmaceutical Data
Remember when all your data lived safely behind your company firewall? Those days are long gone. With over 83% of pharmaceutical companies now using cloud environments, securing data in the cloud has become mission-critical.
“The cloud migration was keeping me up at night,” confessed one of our pharmaceutical clients in Matawan. “We needed to collaborate globally on research, but the thought of our proprietary formulas living on someone else’s servers was terrifying.”
We helped them implement a secure cloud environment that addressed these concerns through multiple layers of protection. This included configuring their systems with least privilege access principles (nobody gets more access than they absolutely need), setting up regular audits of cloud resources, and encrypting all sensitive data stored in the cloud.
Cloud Access Security Brokers (CASBs) play a vital role here too, acting like security guards that monitor cloud usage, detect unusual activities, and enforce security policies across different cloud services. Think of them as your eyes and ears in the cloud, constantly watching for signs of trouble.
Just as important is having a solid backup strategy. We recommend the 3-2-1 approach: keep 3 copies of your data on 2 different types of storage with 1 copy stored off-site. And don’t forget to make some of these backups immutable (unchangeable), which provides excellent protection against ransomware attacks.
AI-Powered Threat Detection and Response
Artificial intelligence isn’t just revolutionizing drug findy—it’s changing how we protect pharmaceutical data too.
Traditional security tools are like fishing with a net: they catch a lot, but you spend hours sorting through false alarms. AI-powered security, on the other hand, is like fishing with a smart harpoon that only targets actual threats.
“AI-powered security tools have reduced our alert fatigue by 80% while improving our detection capabilities,” shared a CISO from a pharmaceutical company in Woodbridge. “We’re now able to focus on the threats that matter most to our critical data assets.”
These AI systems establish baselines of normal behavior for users and systems, then flag anything unusual—like a researcher suddenly downloading gigabytes of proprietary data at 2 AM. They can even respond automatically to contain threats before they spread, patch vulnerabilities, and prioritize alerts based on the risk to your most critical pharmaceutical data.
Perhaps most impressively, these systems can actually predict where attackers might strike next, allowing you to strengthen defenses before an attack even begins. It’s like having a security team that can see the future.
Zero Trust Architecture: A New Paradigm for Pharmaceutical Security
In today’s interconnected world, the old castle-and-moat approach to security (strong perimeter, soft interior) simply doesn’t work anymore. That’s why forward-thinking pharmaceutical companies are embracing Zero Trust architecture, which operates on a simple principle: trust nothing, verify everything.
“Implementing Zero Trust has fundamentally changed how we approach security,” notes a pharmaceutical security director from New Brunswick. “Instead of focusing on perimeter defense, we now secure each data asset independently, significantly reducing our attack surface.”
In a Zero Trust model, you verify explicitly (using multiple factors to authenticate users), limit access rights to the absolute minimum needed, and operate as if a breach has already occurred. It’s a bit like running your lab with the assumption that contamination is always possible—you take precautions at every step, not just at the entrance.
This approach is particularly valuable for pharmaceutical companies because it compartmentalizes data. If attackers do breach one system, they don’t automatically gain access to everything else. Your clinical trial data remains protected even if someone compromises your HR database.
Advanced Encryption Methods for Pharmaceutical Data
Encryption has always been a cornerstone of data protection, but the methods are becoming increasingly sophisticated. Today’s data protection services for pharma include advanced encryption approaches that were science fiction just a few years ago.
Homomorphic encryption sounds complex (and mathematically, it is), but the concept is revolutionary: it allows computation on encrypted data without decrypting it first. Imagine being able to run complex analyses on clinical trial data while it remains encrypted, protecting patient privacy while still extracting valuable insights.
With quantum computing on the horizon (and the threat it poses to current encryption standards), forward-thinking pharmaceutical companies are also implementing quantum-resistant encryption. This ensures that today’s securely encrypted intellectual property—which might include formulations for drugs that haven’t even hit the market yet—remains protected in the quantum future.
Tokenization offers another powerful approach, replacing sensitive data with non-sensitive placeholders. This reduces the footprint of sensitive information in your systems and simplifies compliance with regulations like GDPR and HIPAA.
Managing Third-Party Risks in the Pharmaceutical Supply Chain
The modern pharmaceutical company doesn’t operate in isolation. You likely work with dozens, perhaps hundreds, of vendors and partners—each one a potential weak link in your security chain.
“Our biggest breach didn’t come through our front door,” admitted one pharmaceutical executive. “It came through a small vendor who had access to our systems for inventory management. They had terrible security practices, and hackers used them as a stepping stone to reach us.”
At Titan Technologies, we help our pharmaceutical clients implement comprehensive vendor risk management programs that include thorough security assessments before engagement, contractual security requirements with teeth, regular audits of vendor security practices, strictly limited access to data, and continuous monitoring of vendor activities within your systems.
“We’ve implemented a comprehensive vendor risk management program that includes both initial assessments and ongoing monitoring,” explains our risk management specialist. “For our pharmaceutical clients, this is particularly important given the sensitive nature of their data and the regulatory requirements they face.”
The reality is that your security is only as strong as your weakest link—and in today’s interconnected pharmaceutical ecosystem, those links extend far beyond your own walls.
Learn more about our Data Protection Governance, Risk Management, and Compliance services
Consequences of Non-Compliance and Data Breaches
Let’s face it – when pharmaceutical companies drop the ball on data protection, the fallout isn’t pretty. The consequences of inadequate data protection services for pharma can hit from all sides, creating a perfect storm of problems that can take years to overcome.
Financial Impact: Beyond the Immediate Costs
When a data breach hits a pharmaceutical company, the financial bleeding starts immediately and often continues long-term.
The direct costs add up quickly – regulatory fines that can reach into the millions, legal fees that seem to multiply with each passing month, expensive forensic investigations to figure out what went wrong, and customer notification services that must be provided to affected individuals.
But that’s just the beginning. The hidden costs often pack an even bigger punch – operations grind to a halt during remediation, insurance premiums skyrocket, and security systems need complete overhauls. Perhaps most painfully, business opportunities vanish as partners become hesitant to work with a company that couldn’t protect its data.
A pharmaceutical company in Freehold shared with us their sobering reality: “After experiencing a significant data breach, we spent over $3 million on immediate remediation efforts alone. The long-term costs in terms of improved security measures, legal consultations, and regulatory compliance have been substantially higher.”
With the average cost of a pharmaceutical data breach exceeding $5 million – making pharma the third most expensive industry for breaches – these aren’t hypothetical concerns. And this doesn’t even account for stolen intellectual property, which can represent billions in lost research and development investment.
Regulatory Penalties: A Growing Concern
Regulatory watchdogs have pharmaceutical companies firmly in their sights, and they’re not afraid to bite when they spot violations.
Under GDPR, companies can face penalties up to €20 million or 4% of global annual revenue – whichever hurts more. This isn’t theoretical – a laboratory software provider recently took a €1.5 million hit for a breach affecting nearly 500,000 individuals.
HIPAA violations aren’t any kinder, with penalties ranging from $100 to $50,000 per violation and an annual maximum of $1.5 million per violation category. When you consider that a single breach might involve thousands of records, each representing a potential violation, the numbers become staggering.
The FDA has its own toolbox of enforcement actions, from warning letters that put your company on notice to consent decrees that place your operations under court supervision. They can even seize products or block imports if they determine your data protection practices have compromised product integrity.
As one regulatory compliance expert bluntly put it: “Data breaches are doing more damage than ever. Beyond the immediate financial penalties, the operational disruptions from regulatory investigations can set research and development back by months or even years.”
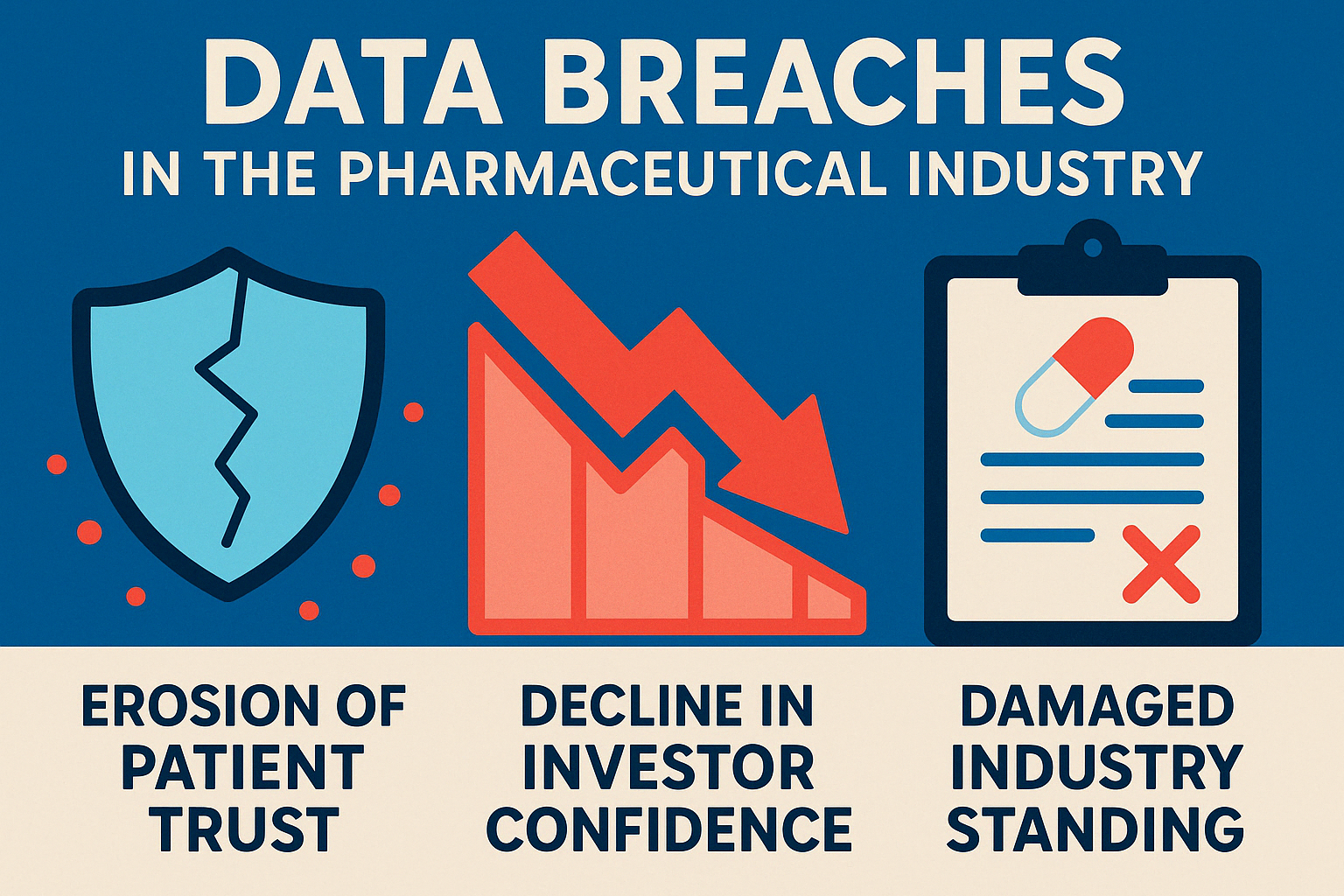
Reputational Damage: The Long-Term Impact
Money and regulatory headaches aside, the most lasting consequence may be the blow to a company’s reputation. In an industry built on trust, this damage cuts particularly deep.
Patient trust evaporates quickly when private health information is compromised. Clinical trial recruitment numbers plummet, and even existing patients may become reluctant to continue treatments or share necessary information with their providers.
Investors respond with equal alarm. Stock prices typically take an immediate hit following a breach announcement, but the real damage comes from the ongoing scrutiny and skepticism from shareholders. Raising capital for new ventures becomes an uphill battle when your company is seen as a security risk.
Within the industry, professional relationships suffer too. Healthcare providers become hesitant to prescribe your medications, research institutions think twice before collaborating, and your standing in the scientific community – built over decades – can erode in months.
A pharmaceutical executive from Elizabeth shared this painful lesson: “After our data breach made headlines, we saw immediate impacts on our clinical trial recruitment. It took us nearly two years of dedicated effort to rebuild patient trust and get our research programs back on track.”
Case Study: The Ripple Effects of a Pharmaceutical Data Breach
To understand how these consequences play out in real time, consider a recent incident involving a state-sponsored cyberattack on a pharmaceutical company:
The trouble began when attackers gained access to research data related to a promising new drug candidate. The company had to immediately suspend clinical trials while investigating how far the breach extended. This alone cost them months of development time.
Multiple regulatory agencies swooped in, launching investigations that required mountains of documentation and hours of executive testimony. The company’s leadership team spent more time in conference rooms with lawyers than running their business.
Shareholders weren’t pleased, filing lawsuits alleging inadequate security measures. The legal battle dragged on for years.
The most painful consequence? Their drug’s development was delayed by 18 months, creating an opening that competitors quickly filled. Their market advantage – the result of years of research – vanished.
When all was said and done, the total costs – including legal settlements, regulatory penalties, and lost market opportunity – exceeded $100 million.
“This case demonstrates why we advocate for a proactive, comprehensive approach to data protection,” our security team at Titan Technologies often explains to clients. “The cost of prevention is always lower than the cost of remediation, especially in the pharmaceutical industry where the stakes are so high.”
The lesson is clear: in today’s pharmaceutical landscape, robust data protection services for pharma aren’t a luxury – they’re a necessity for survival. The question isn’t whether you can afford good data protection; it’s whether you can afford to go without it.
Conclusion: Building a Resilient Data Protection Strategy
The pharmaceutical industry sits at a critical intersection where patient trust, intellectual property, and regulatory compliance converge. As we’ve seen throughout this article, data protection services for pharma aren’t just boxes to check—they’re essential investments in your company’s future.
Key Takeaways for Pharmaceutical Data Protection
The journey to robust data protection begins with understanding what you’re protecting. Comprehensive data mapping gives you visibility into your sensitive information assets, creating the foundation for everything that follows. Without this critical first step, your security efforts might miss your most vulnerable data.
Taking a risk-based approach allows you to focus your resources where they matter most. Your clinical trial data and proprietary research represent years of work and millions in investment—they deserve proportional protection. By identifying your crown jewels, you can allocate your security budget more effectively.
Technology continues to evolve, and so do the threats facing pharmaceutical companies. We’ve seen clients transform their security posture by embracing AI-powered threat detection systems that identify suspicious patterns before breaches occur. Zero Trust Architecture has similarly revolutionized how pharmaceutical companies approach access control, moving beyond the outdated perimeter-based model to a more sophisticated “verify everything” approach.
Clear governance makes the difference between a security strategy that exists on paper and one that works in practice. Whether you choose an in-house Data Protection Officer or partner with specialists like our team at Titan Technologies, having defined ownership of data protection responsibilities ensures nothing falls through the cracks.
Despite your best efforts, security incidents may still occur. We’ve helped clients develop and test incident response plans that dramatically reduced their recovery time and minimized damage when breaches happened. These plans aren’t just technical documents—they’re roadmaps for maintaining business continuity during your most challenging moments.
Finally, your employees represent both your greatest vulnerability and your strongest defense. Creating a culture where everyone understands their role in protecting sensitive information transforms security from an IT department concern to an organizational commitment.
How Titan Technologies Can Help
At Titan Technologies, we’ve spent years helping pharmaceutical companies across Central New Jersey protect their most valuable assets. Our team understands the unique challenges you face, from HIPAA compliance to securing intellectual property.
Our comprehensive data protection services for pharma start with understanding your specific needs. We perform thorough security assessments that identify vulnerabilities before they can be exploited. Our managed security services provide round-the-clock monitoring, giving you peace of mind that someone is always watching for threats.
Navigating the complex web of regulations facing pharmaceutical companies requires specialized expertise. Our compliance support helps you address requirements from HIPAA, GDPR, FDA and other regulatory bodies without disrupting your core operations.
As pharmaceutical companies increasingly move to cloud environments, our cloud security services ensure your data remains protected during and after migration. We configure secure environments that balance accessibility for authorized users with strong protection against threats.
When incidents occur, having a tested response plan makes all the difference. We help develop comprehensive incident response procedures custom to pharmaceutical scenarios, then test them regularly to ensure they’ll work when you need them most.
Your team members need to understand their role in data protection. Our security awareness training programs are customized for pharmaceutical staff, covering industry-specific scenarios and compliance requirements.
With offices throughout Central New Jersey—including Edison, Elizabeth, Lakewood, Newark, Trenton, Princeton, New Brunswick, Matawan, Woodbridge, Freehold, and Red Bank—we provide responsive, local support when you need it most.
Protecting patient data and intellectual property isn’t just about avoiding penalties—it’s about maintaining the trust that allows your business to thrive. When patients trust you with their most sensitive health information, and when investors trust you to safeguard valuable intellectual property, you earn the freedom to focus on your core mission: developing life-saving treatments and therapies.
Learn more about our services and solutions and find how we can help your pharmaceutical organization implement robust, compliant data protection strategies that safeguard your most valuable assets while enabling continued growth and innovation.
Remember: in the pharmaceutical industry, data protection isn’t just a technical challenge—it’s a fundamental business imperative that requires ongoing attention, investment, and expertise. With the right partner and approach, you can turn data protection from a compliance burden into a competitive advantage.